Blog
GLP1 Receptor Agonists: Investigational Agents in Liver Fat Reduction and NASH Progression Models

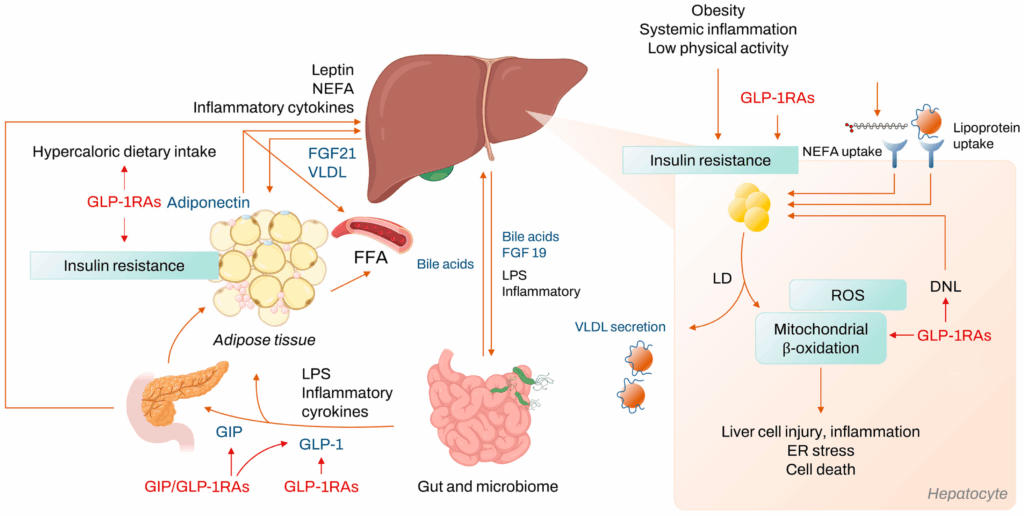
Non-alcoholic fatty liver disease (NAFLD) is among the most frequently studied hepatic disorders, characterized by excessive lipid accumulation in liver cells. Within this spectrum, non-alcoholic steatohepatitis (NASH) presents a more advanced histological profile, marked by steatosis, inflammation, and varying degrees of fibrosis. In preclinical and academic settings, metabolic compounds such as glucagon-like peptide-1 receptor agonists (GLP1 RAs) have been investigated for their potential influence on hepatic outcomes.
GLP1 RAs, such as liraglutide, semaglutide, and dulaglutidewere originally developed for glycemic modulation. However, subsequent laboratory investigations and clinical research have observed secondary effects on hepatic lipid metabolism, insulin sensitivity, and markers of inflammation.

In controlled models, one proposed mechanism involves the modulation of hepatic insulin resistance and suppression of de novo lipogenesis. In insulin-resistant states, excessive nutrient delivery to the liver promotes steatogenesis. Research suggests GLP1 RA exposure may be associated with reductions in intrahepatic lipid accumulation via enhanced insulin signaling and increased lipid oxidation. Published findings from human clinical trials (e.g., Armstrong et al., 2016; Newsome et al., 2021) have shown that GLP1 RAs were associated with improvements in hepatic biomarkers and histological endpoints. These trials investigated the effects of liraglutide and semaglutide in populations with biopsy-confirmed NASH, reporting statistically significant differences in hepatic inflammation scores and steatosis resolution compared to placebo controls.
In addition to lipid modulation, GLP1 RAs have demonstrated anti-inflammatory and anti-fibrotic potential in various animal models. Data suggest a reduction in pro-inflammatory cytokine expression and diminished hepatic stellate cell activation. Improvements in mitochondrial efficiency and oxidative stress markers have also been noted in controlled laboratory contexts. Appetite regulation, a well-documented pharmacodynamic effect of GLP1 RAs, may further contribute to body composition changes that indirectly affect hepatic status in experimental models. As continued research emerges from both academic and clinical sectors, GLP1 RAs remain subjects of interest for their multifaceted roles in metabolic studies. Their full implications in hepatic systems remain under investigation, with future trials expected to provide further insight into long-term outcomes.
Citations
- Armstrong, M. J., et al. (2016). Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN trial). The Lancet, 387(10019), 679–690.
- Newsome, P. N., et al. (2021). A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. The New England Journal of Medicine, 384(12), 1113–1124.
- Nauck, M. A., & Meier, J. J. (2019). Incretin hormones: their role in health and disease. Diabetes, Obesity and Metabolism, 21(S1), 5–21.
- Svegliati-Baroni, G., et al. (2011). GLP-1 receptor activation reduces hepatic inflammation and fibrogenesis. Gut, 60(8), 1104–1113.
- Petit, J. M., et al. (2020). GLP-1 receptor agonists for the treatment of nonalcoholic steatohepatitis: from rationale to clinical proof-of-concept. Therapeutic Advances in Gastroenterology, 13, 1–12.
Disclaimer
This article is intended for educational and informational purposes only. The compounds discussed are sold exclusively for laboratory research use and are not approved by the FDA for human consumption, diagnosis, treatment, or prevention of any disease. No information presented here should be interpreted as medical advice or endorsement of human therapeutic use. Always consult a qualified professional for any health-related questions.
